Scuba diving, a thrilling exploration of the underwater world, relies on a deep understanding of physics, particularly pressure. One crucial concept for every diver to grasp is gauge pressure. Understanding gauge pressure is paramount for safe and enjoyable dives, as it directly impacts buoyancy, air consumption, and depth calculations. It’s the pressure relative to the surrounding atmospheric pressure, and knowing how it works is essential for responsible underwater exploration.
Understanding Pressure in Scuba Diving
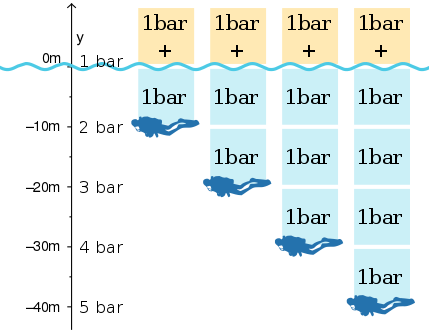
Pressure is a fundamental force in the underwater environment. As a diver descends, the weight of the water above increases, resulting in higher pressure. This pressure affects everything from the air in your scuba tank to the air spaces in your body. There are two main types of pressure to consider: absolute pressure and gauge pressure.
- Absolute Pressure: This is the total pressure exerted on an object, including atmospheric pressure and the pressure from the water column.
- Gauge Pressure: This is the pressure relative to atmospheric pressure. It’s the difference between the absolute pressure and the atmospheric pressure.
Why Gauge Pressure Matters
Gauge pressure is what scuba divers primarily use to measure depth and air consumption. Dive gauges, like submersible pressure gauges (SPGs), display gauge pressure. This is because the gauge is already calibrated to account for atmospheric pressure at the surface.
Consider this:
- At sea level, atmospheric pressure is approximately 1 atmosphere (atm) or 14.7 pounds per square inch (psi).
- For every 33 feet (10 meters) of descent in saltwater, the pressure increases by 1 atm.
Therefore, at 33 feet, the absolute pressure is 2 atm (1 atm atmospheric + 1 atm water), but the gauge pressure is 1 atm. The gauge only shows the pressure exerted by the water column.
How Gauge Pressure Affects Diving
Gauge pressure directly influences several aspects of scuba diving:
- Depth Calculation: Dive computers and depth gauges use gauge pressure to calculate a diver’s depth.
- Air Consumption: Air consumption increases with depth due to the increased pressure. A diver breathes more air at 66 feet (2 atm gauge pressure) than at 33 feet (1 atm gauge pressure).
- Buoyancy: Changes in pressure affect the volume of air in a diver’s buoyancy compensator (BCD). As a diver descends, the pressure increases, compressing the air in the BCD, causing the diver to become less buoyant.
- Equipment Function: Regulators are designed to deliver air at the ambient pressure, which is the gauge pressure plus atmospheric pressure.
FAQ: Gauge Pressure in Scuba Diving
- Q: What happens if I ignore gauge pressure?
- A: Ignoring gauge pressure can lead to inaccurate depth calculations, incorrect air consumption estimates, and potential buoyancy control issues.
- Q: Is gauge pressure the same as absolute pressure?
- A: No. Gauge pressure is the pressure relative to atmospheric pressure, while absolute pressure includes atmospheric pressure.
- Q: How do I read a submersible pressure gauge (SPG)?
- A: SPGs display gauge pressure in either psi or bar. The reading indicates the amount of air remaining in your scuba tank.
- Q: Why is understanding pressure important for scuba diving?
- A: Understanding pressure is crucial for safe diving practices, including managing buoyancy, monitoring air supply, and avoiding decompression sickness.
Advanced Considerations for Gauge Pressure
Beyond the basics, several more nuanced aspects of gauge pressure are important for experienced divers and those pursuing advanced certifications. These considerations often involve more complex calculations and a deeper understanding of diving physics.
Partial Pressure of Gases
While gauge pressure gives us the overall pressure exerted on a diver, it’s also crucial to understand the partial pressure of individual gases within the breathing mix. This is particularly relevant when using enriched air nitrox or trimix. The partial pressure of oxygen, for example, must be carefully monitored to avoid oxygen toxicity. Dalton’s Law of Partial Pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas. Therefore, at a given gauge pressure, the partial pressure of oxygen will vary depending on the percentage of oxygen in the breathing mix.
Decompression Theory and Gauge Pressure
Decompression theory relies heavily on understanding pressure gradients and how gases dissolve into and out of body tissues. As a diver descends and the gauge pressure increases, nitrogen dissolves into the tissues. During ascent, the gauge pressure decreases, and the nitrogen comes out of solution. If the ascent is too rapid, the nitrogen can form bubbles, leading to decompression sickness (DCS). Decompression algorithms use gauge pressure and dive time to calculate safe ascent profiles and decompression stops.
Gauge Pressure and Altitude Diving
Diving at altitude presents unique challenges because the atmospheric pressure is lower than at sea level. This means that the gauge pressure at a given depth will be different than at sea level. Dive computers designed for altitude diving compensate for this difference by adjusting the decompression calculations. It’s crucial to use a dive computer specifically designed for altitude diving or to use altitude diving tables to ensure a safe dive profile.
Comparative Table: Pressure Types
| Pressure Type | Definition | Measurement | Relevance to Diving |
|---|---|---|---|
| Absolute Pressure | Total pressure exerted on an object, including atmospheric pressure and water pressure. | Measured from a true vacuum (zero pressure). | Important for understanding the overall forces acting on a diver and equipment. |
| Gauge Pressure | Pressure relative to atmospheric pressure. | Measured relative to atmospheric pressure (zeroed at sea level). | Primarily used for depth calculation, air consumption monitoring, and buoyancy control. |
| Partial Pressure | The pressure exerted by a single gas in a mixture of gases. | Calculated based on the percentage of the gas in the mixture and the total pressure. | Crucial for understanding gas toxicity risks (e.g., oxygen toxicity) and decompression theory. |
Mastering the nuances of gauge pressure, along with related concepts like partial pressure and decompression theory, is essential for becoming a skilled and safe scuba diver. Continuous learning and practical experience are key to developing a comprehensive understanding of the underwater environment. Remember, safe diving is informed diving, and a solid grasp of pressure dynamics is a fundamental building block for any diver’s knowledge base. Always prioritize safety and seek guidance from experienced instructors to further your understanding of the complexities of scuba diving.